FORTIFY YOUR MEDICAL DEVICE CYBERSECURITY STRATEGY AGAINST EVOLVING THREATS
Defending against cyberattacks is a daily reality for your health system. Cyber-criminals using sophisticated and advanced technologies like artificial intelligence pose a growing threat to patient care and safety. These attacks drain financial resources, erode reputation & patient trust, and cripple clinical operations. Cybersecurity vulnerabilities can be found in every aspect of healthcare technology, including medical devices.
Increase in ransomware attacks on healthcare delivery organizations (2021-2023)¹
of validated remediations (by make, model, description) received from the OEMs by TRIMEDX³
The risks to medical device cybersecurity are increasing dramatically as hospital inventories become more integrated with online networks. With this trend, the link between cybersecurity, medical device maintenance, and truly effective capital planning has grown more important than ever. While biomedical engineering teams and IT departments have often operated separately, a much more coordinated effort is now essential. Hospital administrators, CIOs, CISOs, and biomedical technicians are all forced to take an active role in understanding healthcare medical device cybersecurity threats to help protect their organizations.
Bridging the Visibility Gap

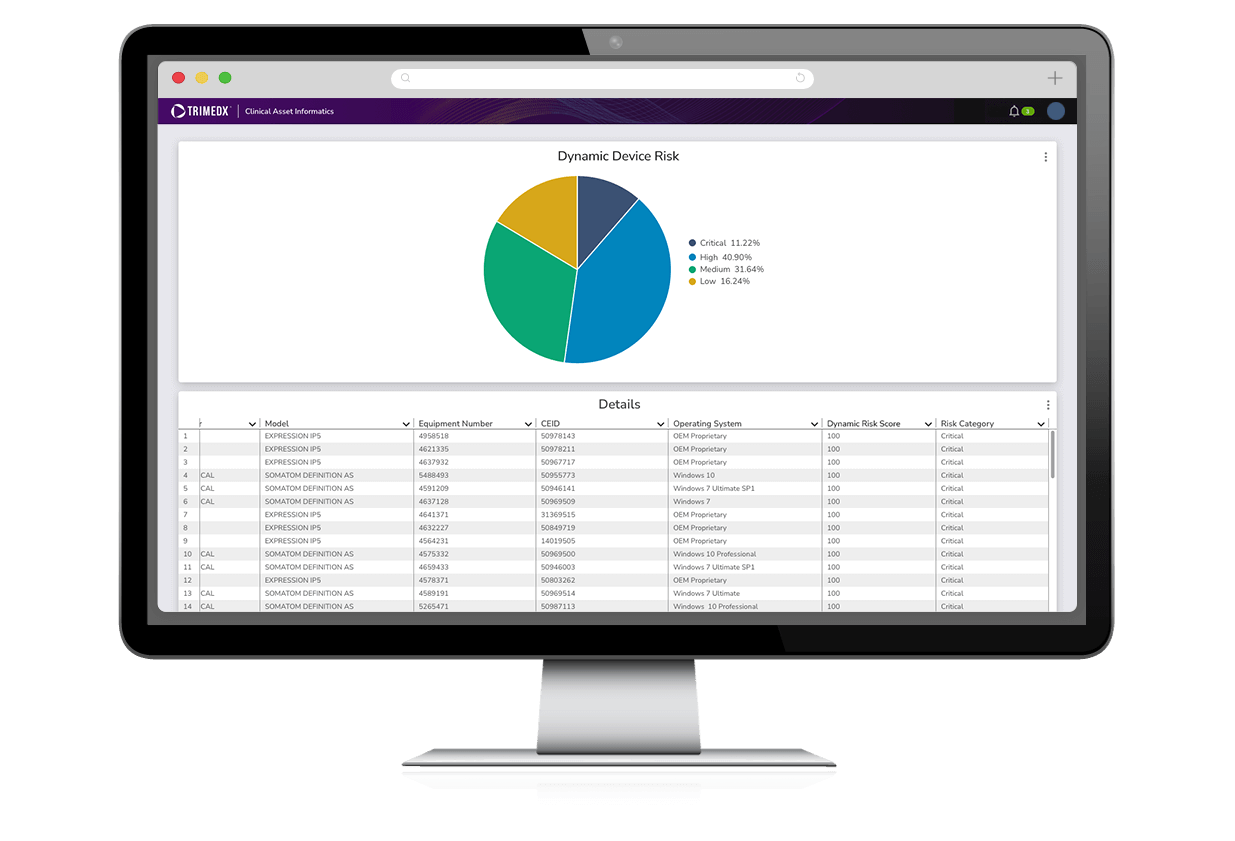
 A true clinical asset management strategy must incorporate a comprehensive understanding of medical device inventories, the relevant vulnerabilities for each device, and the risk these vulnerabilities pose. Combining full visibility with standardized processes drives efficient remediation and capital planning, bringing clinical engineering, IT, and finance together.
A true clinical asset management strategy must incorporate a comprehensive understanding of medical device inventories, the relevant vulnerabilities for each device, and the risk these vulnerabilities pose. Combining full visibility with standardized processes drives efficient remediation and capital planning, bringing clinical engineering, IT, and finance together.
The TRIMEDX CYBER solution combines our expertise in maintaining and managing medical device inventories with expansive data on cybersecurity vulnerabilities, real-time monitoring, dynamic risk scoring, and remediation of cyber vulnerabilities. You can quickly identify a vulnerability and, just as importantly, know what you need to do to resolve it with this integrated cybersecurity ecosystem and real-time monitoring, management, and remediation. TRIMEDX data and technology, an expert CYBER support team, and cybersecurity training mean you can consistently monitor vulnerabilities, quickly identify unusual behavior, and implement remediation strategies for true closed-loop remediation.
WHITE PAPER
ESTABLISHING A MATURE MEDICAL DEVICE CYBERSECURITY PROGRAM
Health systems are devoting more resources than ever to protecting themselves against cyberattacks. However, a mature cybersecurity program must incorporate robust processes for managing medical devices, which can be a major security blind spot in healthcare. Health systems can apply specific strategies with their device inventories to build a stronger foundation for cybersecurity maturity and make resources more impactful in protecting their patients, staff, and facilities.

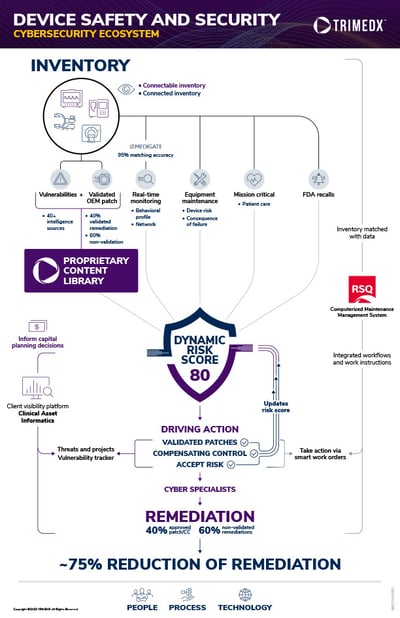
Medical Device Inventory and Risk Monitoring
The TRIMEDX CYBER solution capitalizes on our Clinical Engineering solution to build a thorough understanding of the unique risk factors that are influenced by a health system’s inventory and operational needs.
- Equipment maintenance: The risk to a device from a vulnerability and the potential consequence of failure
- Mission critical status: The risk a device failure or breach poses to patient care activity and the potential impact on patient safety
- FDA alerts and recalls: TRIMEDX’s integrated support functions include notifications for FDA alerts and manufacturer recalls, including automated work orders where necessary, as well as potential cybersecurity impacts
We also employ some of the most comprehensive resources for identifying vulnerabilities and monitoring anomalous behavior.
- Real-time Monitoring: Our technology partners integrate directly with the TRIMEDX CYBER solution to provide advanced behavioral profiles and advanced monitoring of network-connected devices with AI
- Proprietary content library: A continually updated compilation of cybersecurity vulnerabilities and validated OEM software patches
Dynamic Risk Score

Driving Responsive Remediation
The integration of TRIMEDX’s experience in clinical engineering and robust medical device cybersecurity intelligence also creates opportunities to develop and execute confident, efficient strategies to remediate vulnerabilities.
CYBER training equips TRIMEDX associates with crucial knowledge to contribute to cybersecurity even in a rapidly evolving landscape of threats. Continuous education ensures that technicians can act effectively on the data that TRIMEDX technology delivers.
TRIMEDX CYBER analysts


Leveraging Clinical Asset Informatics